INTRODUCING THE PASS FRAMEWORK AND ITS IMPLEMENTATION IN FIRST-YEAR GENERAL CHEMISTRY HIGHER EDUCATION
General chemistry higher education students who have the option of timely formative feedback loops when practicing homework questions is an essential element in supporting the development of understanding and applying key concepts in foundational chemistry. When students do not have access to an instructor, having an automated framework to guide their learning process can meet their needs in a personalized way. While open educational resources (OER) have provided the content and practice questions for general chemistry courses, how students apply the key concepts, contextualize the learning contexts, connect to foundational theory, and demonstrate their understanding had the potential to be explored in more detail. The development of the platform adapted/adaptable strategic solutions (PASS) framework is introduced as an example of a literature-based formative assessment approach that consistently presents comprehensive solutions to practice problems for students engaging with OER. This open framework can be used to help instructors develop general chemistry solutions for use in multiple digital platforms and to use more OER in their courses. The example solutions follow a scaffolded template designed to allow students to choose the level of support they need as they work through practice questions, which provides automated and equitable formative feedback and guidance for all self-directed and self-regulated students completing chemistry solutions independently.
KEY WORDS: formative assessment, self-directed learning, inclusive instructional design, open platforms, OER, higher education, chemistry education, scaffolded solutions, solution template
1. INTRODUCTION
The platform adapted/adaptable strategic solutions (PASS) framework is a literature-based formative assessment approach that consistently presents comprehensive solutions to practice problems for students engaging with open educational resources (OER). The open PASS framework (https://chempass.opened.ca/) is a self-directed model that can be used by educators, instructional designers, content creators, and learners to systematically organize the required, relevant, and supplementary information related to solving both quantitative and qualitative style general chemistry questions.
The PASS project aims to support students in their individual formative assessment, when outside the classroom and learning independently, with scaffolded solutions for open homework problems. Scaffolding provides learners with an appropriate level of assistance to build understanding and skills they cannot yet accomplish alone (Lipscomb et al., 2010; Yuriev et al., 2017).
In this paper we present the PASS framework and discuss the rationale and utility of each component as it integrates pedagogy and practice. Using our collection of general chemistry PASS templates, we will illustrate how to implement the framework across a range of media, modalities, and open platforms (i.e., printable worksheet, learning management system (LMS) quizzes, H5P quizzes, videos, H5P embedded videos, Pressbooks, and LibreTexts) to address the potential of formative assessment in higher education contexts. Finally, we propose further development and future uses of the PASS approach. These examples include adoption by educators and creators outside the chemistry discipline and validation of student experience and efficacy.
1.1 Background (Existing Gap Challenges in STEM/First-Year General Chemistry)
There are multiple challenges in first-year science courses, including chemistry. These gateway courses are typically high-enrollment, high-risk, introductory postsecondary courses (Koch, 2017). Success in these courses allows students to continue in a program, whereas failure may result in attrition (Flanders, 2017; Weston et al., 2019). First-year general chemistry is a required course for many science programs and professional schools, including those in life sciences. A lack of success or understanding in first-year general chemistry courses has been reported to be a key cause of students leaving programs which require this course (Barr et al., 2010; Harris et al., 2020).
To facilitate student success in chemistry and build deeper understanding of course content, practice questions can task students to solve a problem outside of the classroom and be used formatively or summatively. The goal of practice questions is to provide opportunities for students to be able to use theory and connect their knowledge to determine how to develop a solution to tasks that derive from or connect with theory. Practice questions become homework when they are assigned by an instructor and may or may not have a course grade attached to them. Chemistry practice questions can have a wide range of question types, from quantitative problems requiring use of formulas and calculations, to qualitative problems with one or more solutions, to problems that require approximations or assumptions or those that have no unique solution (Tsaparlis, 2021; Wood, 2006).
Completion of chemistry homework questions have been reported to support studying behaviors and correlate positively with course performance (Leinhardt et al., 2007; Richards-Babb & Jackson, 2011; Bowman et al., 2014). First-year general chemistry students require deliberate practice with immediate and thoughtful feedback (Algar et al., 2022; Herrington, 2018). Examples of summative or formative assessments can include practice problems that are introduced in many ways by first-year general chemistry instructors, including as pre- or postclass assignments, as timed assessments (Shi et al., 2024; Wang et al., 2024). Providing an appropriate level of support to first-year general chemistry student learners outside of the classroom using technology has been shown to build confidence and strengthen problem-solving skills (Herrington, 2018; Mahalingam et al., 2019). Students need to develop reasoning skills to be successful at solving conceptual chemistry problems (Cracolice et al., 2008).
When analyzing the impact of student time spent on first-year general chemistry practice problems Gondra et al. (2024) found that mental and emotional fatigue affected problem-solving success. To combat this, they recommended instructors adopt pedagogical strategies to help students recognize problem-solving is iterative and takes time, which develops understanding, and that emphasizing critical parts of problems to guide selection of the best approach to solve will help students gain confidence and developed resilience (Gondra et al., 2024). Practice questions are an essential element for all students to improve their chemistry learning.
Students choose to complete practice questions for a variety of reasons, including receiving marks, increasing confidence, and improving understanding (Salame & Hanna, 2020). A major component of the PASS framework considered the need for better course design and support for underrepresented students in science, technology, engineering and mathematics (STEM) and chemistry, including women, minorities, and first-generation university students (Ryu et al., 2021). As universal design for learning (UDL) (Almeqdad et al., 2023) advocates, we recognized that (1) improved skills builds confidence, which improves engagement and grades, especially for underrepresented students (Fink et al., 2018, 2020; Tashiro & Talanquer, 2021); (2) confidence in a specific content area can be improved by practice (Harris et al., 2020); and (3) things that improve skills (like practice and flexible modifications) are of benefit to all students for different reasons (Lee & Orgill, 2022; Salehi et al., 2020; Siegel et al., 2008; Stone, 2021). Our goal was to develop PASS with underrepresented students as our leading personas to design for equity and diversity as a foundational piece for student-centered teaching.
1.2 Current Literature to Support Context
Traditional assessment practices tend to focus on sharing information and evaluating what the student heard or understood from the information shared. Some common examples of shared chemistry information include textbook readings and questions and answers at the end of a chapter in a textbook. As a result, we felt there was a gap in the learning design approach that considered how the information was being shared and how students were given the opportunity to understand and clarify the information based on their personal understanding of the topics. There were limited ways in which we could track evidence of the step-by-step process students take when analyzing and figuring out chemistry problems. We wanted to design a way to better understand what our students were thinking and where they were running into challenges when working independently. One-on-one support is not feasible in all contexts because of the difficulty in our ability to reach out to each student individually to determine their unique set of skills and weaknesses and the specific area(s) in which they require support. Instead, we opted to design a framework that would comprehensively cover all aspects of the question to guide students to reach the solution.
Based on shared lived experiences in teaching practices that tried to support a diverse range of students in office hours, we noticed the importance of a consistent iterative approach to guiding students through the process of solving practice questions. This process included various promptings of contextual clues, theoretical considerations, hints, and caution against common mistakes. We looked to the literature to connect our shared professional learning design experiences with current learning and pedagogical literature. The PASS framework was designed based on current literature which provides individualized or automated digital feedback that could be adapted or modified for digital platforms to provide specific information and timely feedback based on student needs. The following literature summarizes some of the current learning contexts that describe how other educators have attempted to bridge the gap between one-on-one feedback and support from an educator and/or automated digital feedback, providing students with expertise and support in a timely accessible fashion.
2. FORMATIVE ASSESSMENT: FEEDBACK WHILE LEARNING
As chemistry educators, we hope that students can analyze and evaluate their problem-solving process when completing practice questions. Parmigiani et al. (2024) described a recent study commissioned by the European Commission which underscored that “formative assessment needs to place the learners themselves at the center of the learning and assessment processes, taking a more active and central role both as individual, self-regulated learners and as critical peers” (European Commission: Directorate-General for Education, Youth, Sport and Culture [DG EAC] et al., 2021, p. 7). The methods we have traditionally used to teach chemistry concepts have included lectures, textbook questions and answers, professor and/or teaching assistant meetings, and/or seminars. We designed the PASS framework to expand upon these approaches by considering formative assessment strategies.
Formative assessment is the feedback students and instructors use to check for understanding while being engaged in the learning process. It aspires to support learning, while summative assessment only recognizes a snapshot of student performance (Crossouard & Pryor, 2012). Feedback is information given to learners regarding their skills or understanding around a task with the goal of modifying their thinking and improving their learning (Buhr, 2020; Hattie & Timperley, 2007; Mandouit & Hattie, 2023). Feedback is used to address a “gap” between students' current level of understanding and their desired level, which is the intent of feedback (Hattie & Timperley, 2007; Sadler, 199). In practicing questions and mastering chemistry skills and competencies, Hattie and Timperley (2007) suggest that there are four types of feedback, focused on the task, process, self-regulation, and individual, which have different purposes and variable impacts on students' learning and different strategies for effective implementation.
2.1 Theoretical Learning Theory Connections
The PASS framework was guided by the following pedagogical theory that supported the development of the learning design.
2.2 Open Educational Practices
Having the opportunity to access codesigned OER that provided step-by-step guidance on how to complete a chemistry equation in an open digital platform that can be expanded upon, communicated with, adopted and adapted by anyone is an example of an open educational practice (OEP). Cronin (2017) defined OEPs “as collaborative practices that include the creation, use, and reuse of OER, as well as pedagogical practices employing participatory technologies, and social networks for interaction, peer-learning, knowledge creation, and empowerment of learners” (p. 18). The PASS framework collaboratively creates OER by sharing the Creative Commons licensed examples, then adapts them in multiple mediums and contexts using an open-learning design. In collaboration with chemistry faculty, instructional designers, student researchers, and librarians, the PASS framework also highlights the importance of open-learning design (Roberts, 2022) by demonstrating the potential of using OEP to codesign formative assessment opportunities for chemistry students.
2.2.1 Self-Directed Learning
The PASS framework highlights the potential for students to have choice in what they learn and how they learn it, a principle of self-directed learning (SDL). Knowles defined SDL as, “A process in which individuals take the initiative, with or without the help of others, in diagnosing their learning needs, formulating learning goals, identifying human and material resources for learning, choosing and implementing appropriate learning strategies, and evaluating learning outcomes” (Knowles, 1975, p. 18). The completion of homework and practice questions is sometimes guided by the weighting of homework completion based on completed grades. In the PASS framework, the goal is to increase a student's intrinsic desire to complete practice questions because they will reflect upon and clarify what they already know and what they have yet to learn or need more clarification about. The PASS framework provides direct connections to content and explanations based on the student's desire to choose more or less support.
2.2.2 Self-Regulated Learning
What makes the PASS framework student-centered is a design that supports a student's intrinsic motivation and offers choices to support their personalized learning. Self-regulated learning (SRL), as defined by Butler and Winne (1995), “is a style of engaging with tasks in which students exercise a suite of powerful skills: setting goals for upgrading knowledge; deliberating about strategies to select those that balance progress toward goals against unwanted costs; and, as steps are taken and the task evolves, monitoring the accumulating effects of their engagement” (p. 245). The PASS framework demonstrates adaptive learning by focusing on the student as the primary agent who chooses if they need additional help or not, and how they want to receive the support. The instructor can choose to expand upon the PASS framework by integrating digital software to help the student make their choices.
2.2.3 Adaptive Learning
When PASS is used in digital contexts to expand personalized student-centered learning opportunities, the pedagogical lens extends from self-directed to more adaptive learning contexts. Adaptive learning is often offered in digital contexts by LMSs to provide timely feedback to students based on their individual skills, abilities, and needs. Adaptive learning is used to assess a learner's understanding of a topic by comparing and contrasting learner data, then providing differentiated and individualized activities for each learner based on what knowledge and skills they have demonstrated and learner preferences. Learners are then provided specific guided activities to help guide them to master specific learning outcomes (Saba & Shearer, 2017).
Our approach expands from current adaptative learning techniques as it integrates mastery learning (ML) (Block & Burns, 1976) and just-in-time learning (JITL) (Killi & Morrison, 2015), without reliance on an automated digital response. ML ensures that students acquire specific skills, that they can demonstrate and practice integrating the specific skills, and that they know they can replicate and apply the specific skills. Within a chemistry practice question context, there are specific discipline-focused skills that need to be mastered, acquired, and demonstrated, and specific ways in which learners can demonstrate their learning. JITL promotes a learning design that intentionally considers a balance between instructor-led consumption of knowledge and student-directed learning, which enables the learner to make timely connections to course content or to their instructor's notes and explanations. Finding the connection to course content easily and quickly supports the student in their understanding of the topic. When completing chemistry practice problems, students must be able to access supplementary information quickly to complete the questions effectively and by themselves.
In our experience, we wanted to design feedback loops that promoted and encouraged student choice. As a result, we wanted to move beyond a traditional responsive system that would only distinguish correct and incorrect answers for students. Instead, we wanted to design the solutions for the practice questions to expand upon adaptive learning considerations (Richards-Babbs et al., 2018). These solutions would provide response-specific feedback so students could understand what they were doing incorrectly and where to find more information to help them understand why it was incorrect, as well as provide commentary to learn why each step was taken in the correct approach.
2.3 Homework Systems in Chemistry as an Example of Existing Adaptive Learning
Homework systems utilize adaptive and personalized learning options to highlight the reinforcement of classroom concepts through supports for student practice, self-reflection, and self-assessment. They are also referred to as adaptive learning platforms, personalized learning platforms, or digital tutoring systems (Lalonde, 2020). Online homework systems may include levels of interactivity, reflective prompts, and guidance, as well as incorporate digital tools that can grade questions, track formative practice, or assess examinations. They are increasingly used in first-year general chemistry (Mahalingam et al., 2019; Richards-Babb & Jackson, 2011; Salame & Hanna, 2020; Wilson & Kennedy, 2017) to provide practice opportunities for students. There are many online chemistry homework systems such as Mastering Chemistry (https://www.pearson.com/en-ca/higher-education/productsservices/mastering/chemistry.html), Aktiv Chemistry (https://aktiv.com/), ALEKS (https://www.aleks.com), and Alchemy (Algar et al., 2022). Online homework systems are considered attractive, as they allow instructors to provide students with formative assessment tools outside of the classroom, students get immediate feedback on an answer, and they increase time students spend on content (Richards-Babb & Jackson, 2011; Wilson & Kennedy, 2017). Current literature supports commercial education/online homework systems by describing their benefits and challenges to potential student learning (Salame & Hanna, 2020; Eichler & Peeples, 2013; Nabulsi et al., 2021), but white papers, reports, and data summaries tended to lack theoretical frameworks and foundations to build and share knowledge. While there is evidence that shows student performance increased for those who used existing homework systems (Salame & Hanna, 2020; Eichler & Peeples, 2013; Nabulsi et al., 2021), students that fall into particular demographic groups may respond more or less positively to these marks-based interventions. For example, Richards-Babbs & Jackson (2011) found gender impacted attitudes toward the use and benefits of online homework systems. While online homework systems are often used for marks, we sought to develop a framework that can be used to develop intrinsic engagement with and understanding of discipline-focused concepts.
3. FRAMEWORK DEVELOPMENT PROCESS
The PASS framework seeks to provide a design that expands upon the literature to support students' nonpunitive exploration for formative self-assessment and equitable practices by presenting students with comprehensive solutions to practice problems.
3.1 Design Considerations and Institutional Contexts
Our focus was to develop a resource that would support all of our students. Our university is an open admission (Williams & Wendler, 2020) institution where admission to first-year general chemistry requires successful completion of either high school chemistry grade 11 or grade 12. There is no prerequisite grade requirement, which means the range of experiences and backgrounds in terms of prior content knowledge is quite diverse. Students struggle with content and concepts, and faculty spend significant time outside of the classrooms supporting student learning.
This project began with a search for open homework questions accompanied by solutions suitable for first-year general chemistry students to use for self-formative assessment. Use of OER is increasing in Canada (Craig, 2020) and around the world (Marín et al., 2022), and is often driven by student complaints about the cost of traditional textbooks (Jhangiani & Jhangiani, 2017). As one of the top five leading open textbook-adopting institutions in British Columbia (Open Textbook Stats – BCcampus OpenEd Resources), our institution encourages the adoption of OER and OEPs.
Guided by our Faculty of Science sustainable practices mandate, Thompson Rivers University's (TRU's) First-Year Chemistry Team began using free open textbooks for all first-year courses in 2022–23. Our OERs were published on the LibreTexts platform, which allows for sustainable adoption and adaptation of shared material with ongoing ability to revise and add new open content. Our LibreTexts include practice exercises at the end of every chapter, similar to the way exercises are presented in a traditional textbook. While most OER practice questions have answers, we recognized that very few had detailed solutions. The lack of instructor ancillary resources, including practice questions, is mentioned in the literature as one reason for not adopting an open textbook (Lantrip & Ray, 2020; Jhangiani et al., 2016; Taylor & Taylor, 2018). While students have provided informal feedback that they appreciate the removal of the cost barrier for a necessary and supportive educational resource, they also wished that the resources provided answers and detailed solutions. While checking an answer provides students with basic feedback, it does not instruct them how to solve the problem, identify the step or steps in the process that require correction, or explain the chemistry theory and rationale that supports the exercise. These are critical pieces in developing a deeper understanding of the material and building confidence outside of the classroom.
3.2 Identifying a Gap in Chemistry OER
Based on the student anecdotal feedback and our acknowledgment that students require more than just an answer to improve their confidence in learning, we set out to find and collect open homework questions (practice questions and exercises that can be used for homework/study outside the classroom) for chemistry that had detailed solutions in order to share them with our students. Unfortunately, we were unable to find anything that met our needs. Commercial publishers offer instructors textbooks, solutions manuals, and interactive activities to support learners as they complete their courses, but students not only have to purchase the textbook, but also pay additional funds or a higher overall rate to include the online homework platforms this requires (Lalonde, 2020).
To bridge this gap, we decided to create our own set of solutions for the open practice exercises we had incorporated into our LibreTexts. We did not intend to compete with the time and energy commercial publishers spend creating interactive activities for students. Instead, our goal was to develop a consistent approach to organizing and presenting answers and detailed solutions for homework practice questions. The design facilitates the inclusion of explicit and exhaustive feedback for students to engage with based on their dynamic learning needs.
3.3 Framework Design
The PASS framework was created by an interdisciplinary team that included an instructional designer and two chemistry educators from an undergraduate-focused, postsecondary institution. The initial audience and application was for undergraduate students studying first-year general chemistry. As a team of discipline-focused and open-learning design experts, we blended aspects of our practical teaching experience with key learning theory concepts and philosophical approaches with the goal of creating a resource to foster effective student skill development.
We synthesized our standard practical techniques of supporting/guiding students in their approach to finding the answer to formative (e.g., practice problems) and summative (i.e., quizzes, term tests) question types. We wanted to expand the traditional solutions available for students. After reviewing the design of the collected open practice exercises, we considered how we could incorporate timely and constructive feedback to our students within the solution. Informed by our literature review, we considered the challenges students faced when completing practice questions outside of class, which highlighted students' prior learning experiences, organizational issues, diversity, language and cultural contexts, and self-confidence (too much and too little). We developed a consistent solution outline that would be able to be applied to all our solutions in order to support student practice and learning. We wanted to incorporate the “teacher-talk” (feedback) (Chin, 2007; Johnston, 2020; Johnson, 2022; Khoza, 2016) that we would use to guide students in solving a problem if we were together in an office hour. By scaffolding the solution, we also felt that a student could then choose the level of support they wished to have as they worked through the problem. Our initial goal was to create a template that could organize newly created solutions into a consistent systematic approach that would provide support to students with a variety of skill and confidence levels in first-year general chemistry. The literature-based framework we developed called PASS (Platform Adaptable Strategic Solution), to support diverse student learning (Fink et al., 2018, 2020; Tashiro & Talanquer, 2021), is intended to model the experience a student might have in an office hour appointment. Here the instructor might test the student's understanding of a concept by first, asking them to solve the problem on their own; second, provide some general instructions or steps to approach the problem; next, show the step-by-step solution to reach the answer; and finally, most importantly, supplement each step with the chemistry context and theory along with hints and reminders to ensure that the students avoid common mistakes. Figure 1 demonstrates the structure of the PASS design.
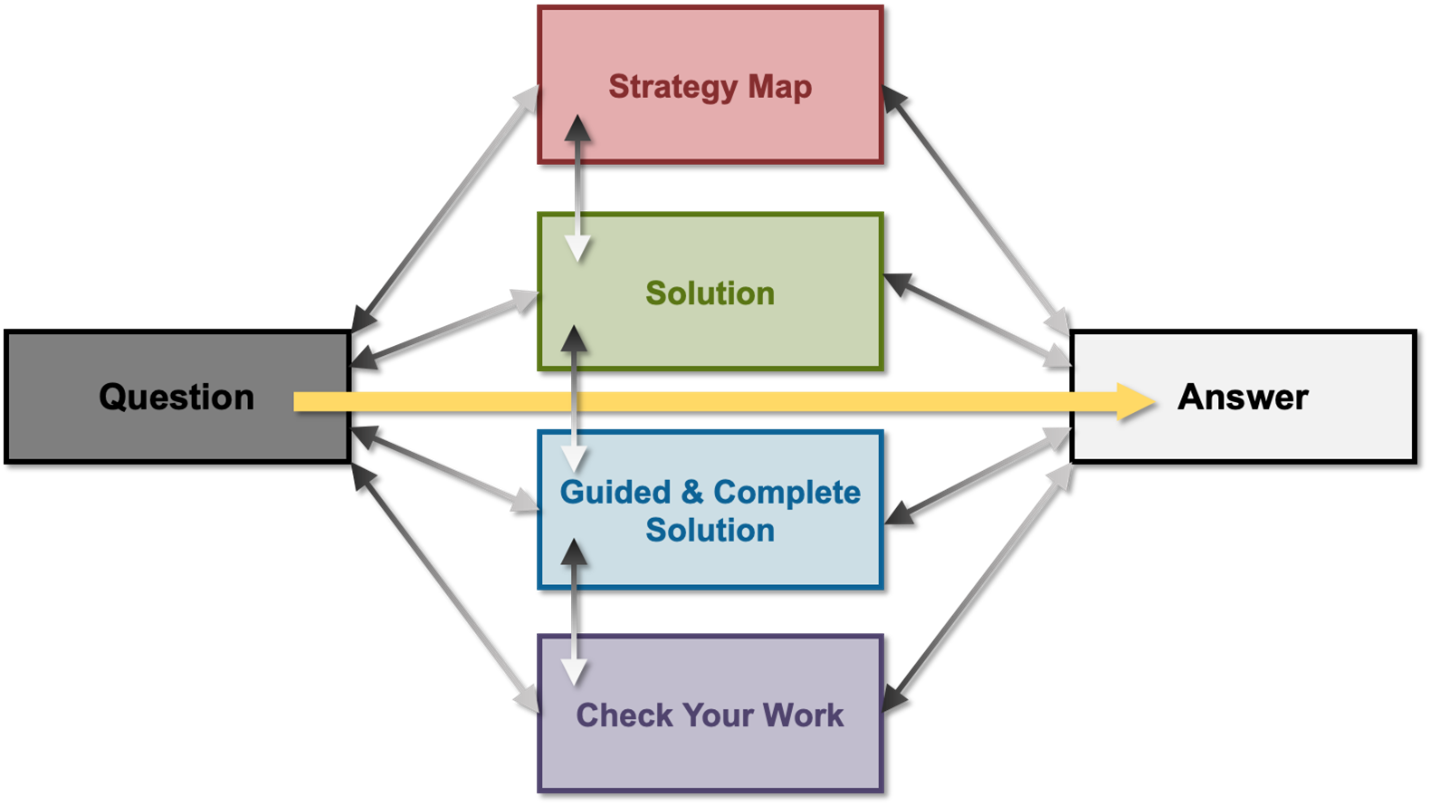
FIG. 1: A visual representation or flow chart displaying the varying paths students may follow as they move from reading the question to finding the answer. Students may or may not require additional support to understand the process of solving the practice problem. As a student moves from “Strategy Map” to “Solution” to “Guided & Complete Solution” (i.e., top to bottom), an increasing level of detail is provided to better comprehend the related theory behind each step.
4. THE PASS FRAMEWORK
The PASS framework is intended to support educators in organizing a comprehensive solution for practice questions/exercises. The blank template is a table in a word processing document (i.e., Microsoft Word), as illustrated in Figure 2 and available online at https://chempass.opened.ca/pass-digital-artifacts/.
Figure 2 is split into two columns with two parts distinguished horizontally, from left to right. The left column is designated for required information or guidance needed by the student to answer the question, whereas the right is intended to provide space for optional supplementary information, such as hints, tips, common errors to watch out for, additional context, another way to think about the topic, or reference to additional resources. Vertically, the table is segmented into sections in the following order: Question, Answer, Strategy Map, Solution, Guided Solution, Complete Solution, Check your Work, and Attributions. Within each section, the table can accommodate as many or as few rows as necessitated by the suggested steps required to solve each specific question. Rows are intended to define discrete ideas or steps used in finding or reconciling the answer, and each idea is presented in a proposed logical order.
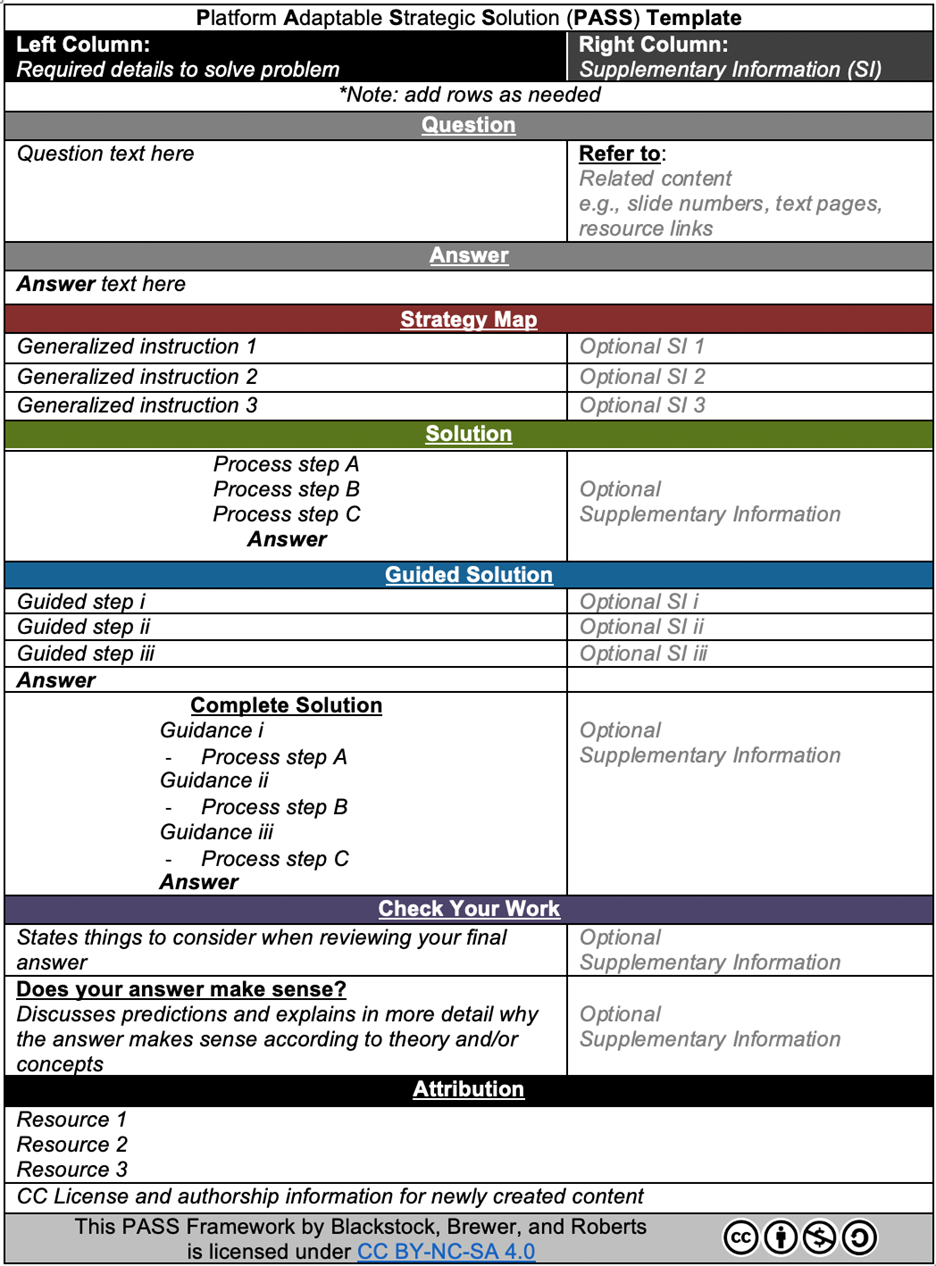
FIG. 2: A blank PASS template. This tool is used by the content creator, professor, or student to consistently organize the details related to solving the question.
Finally, PASS templates (i.e., completed comprehensive solutions) were created for a range of openly sourced representative first-year general chemistry practice questions. The systematic organization of the templated PASS content was shown to be adaptable to be presented in a variety of modes (i.e., .pdf worksheet, LMS quiz, H5P quiz, YouTube video, and H5P video quiz, https://chempass.opened.ca/). Additionally, the PASS templates have been implemented into two digital platforms, LibreTexts and Pressbooks. The utility of each “site” with respect to the intended use of the PASS framework is described and compared below in Section 4.2, later in this article.
4.1 Application: How the Framework Works
In this section we will describe the intention and utility of each component of the template with respect to its use as an organizational content creation tool for educators and as a self-directed formative assessment resource for learners. We also review each segment of a PASS template example completed for a first-year general chemistry practice problem on the topic of gases.
The question is always provided first (Figure 3), with no other details, to encourage the students to formatively assess their understanding and to not corrupt/bias/cloud – wrong word their true ability. Encouraging the student to attempt the question without support better reflects a summative assessment scenario.
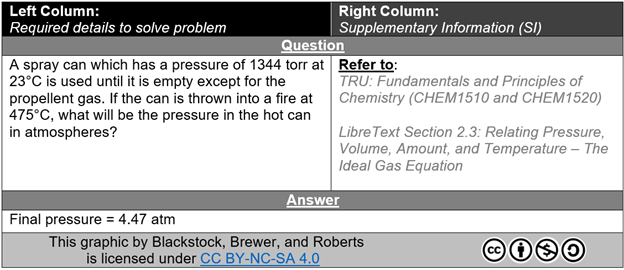
FIG. 3: “Question” and “Answer” excerpt from a completed PASS template example. This is a first-year general chemistry practice problem relating changing conditions of an ideal gas.
We recommend that students attempt the question on their own and check the answer. Ideally, if they are correct, they can move on to the next question. However, many chemistry students do not begin their study practice with mastery. Students may face challenges even at the onset of attempting a question, which leads to a destruction of confidence and self-efficacy.
4.1.1 Strategy Map
If the students do not Figure out the answer, a “strategy map” is provided. The strategy map provides a generalized set of steps suggested to tackle the question but does not instruct the student with exactly what to do. If a student needs assistance with which general steps to take, this section of the PASS template will provide the student with a “spoiler-free” nonspecific procedure (instructions) to follow to reach the answer. This broad direction encourages students to reevaluate the initial question and reattempt. This process seeks to reinforce SRL. Within this strategy map, hints, links, or directions to other resources may be supplied to the student (see Figure 4 for more details).
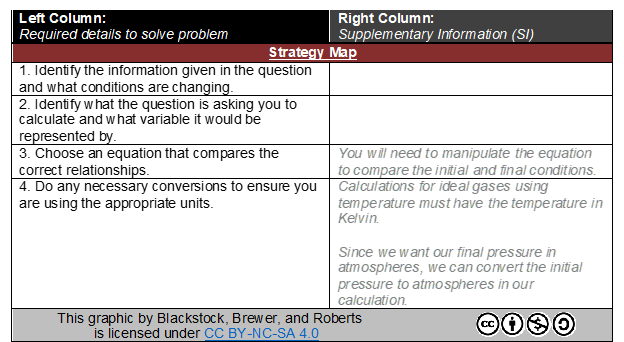
FIG. 4: “Strategy Map” Excerpt from a completed PASS template example. This segment of the PASS template suggests a generic set of instructions to assist the learner in planning their approach to solve the problem without revealing the solution. The learner must still adapt and apply the strategy to the specific details provided in the question.
4.1.2 Solution
The “Solution” is presented next (Figure 5). Spoiler-alert!—This section shows each distinct step of the solution taken to reach the answer without any additional commentary. For a quantitative style question, this eliminates any uncertainty in the mathematical steps, which is often a barrier for first-year students (Powell et al., 2020). For a qualitative style question, this part of the solution supplies the logical flow of relevant theory beyond simple memorization.
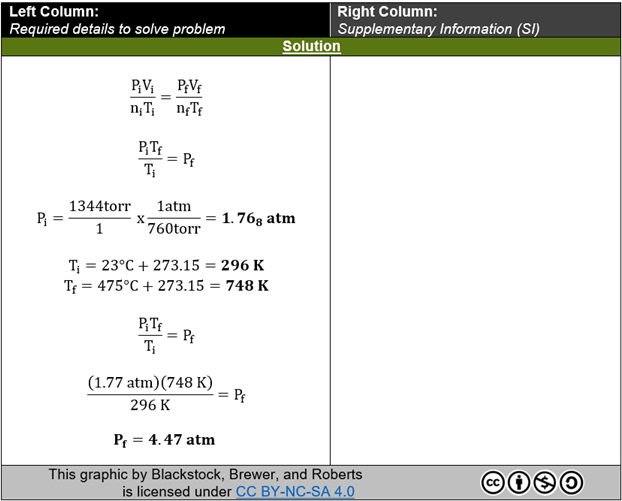
FIG. 5: “Solution” excerpt from a completed PASS template example. This component of the PASS template provides the learner with each step required to reach the answer. No context, comments, or considerations are included as supplementary content in this section.
4.1.3 Guided Solution
Next, the “Guided Solution” is offered (Figure 6) to explain the relevant theory, context, or rationale behind why each step in the solution is performed.

FIG. 6: “Guided Solution” excerpt from a completed PASS template example. Content contained within the left column describes the nature of each step in the solution. Commentary provided in the right column provides the context as to why each step is being performed with respect to core principles in chemistry theory. Additionally, the right column houses helpful tips and common mistakes to consider.
The guided solution's purpose is to replicate the experience a student may have during an effective office hour or lecture, to work through a problem supported by “teacher talk.” In this section you will also find hints embedded throughout that encompass links to read more from supplementary resources, additional contexts, or an alternative perspective, reminders about various unit conversions or significant figures, and common mistakes to avoid.
4.1.4 Complete Solution
Following the guided solution is the “Complete Solution” (Figure 7) that combines the step-by-step solution with the key context points from the guided solution.

FIG. 7: “Complete Solution” excerpt from a completed PASS template example. This section of the PASS template synthesizes the discreet details of the solution with the fundamental rationale behind each step which was described in the previous presentation of the guided solution.
4.1.5 Check Your Work
Finally, the “Check Your Work” section completes the solution in the PASS template (see Figure 8). Mistakes can often be avoided by thinking critically about the theory related to the problem and predicting what the answer should or should not be. This section provides the rationale of why your answer “makes sense” with respect to the chemistry theory. Gilbert (2017) emphasizes that it is essential to use this thought process to avoid making mistakes in similar problems.
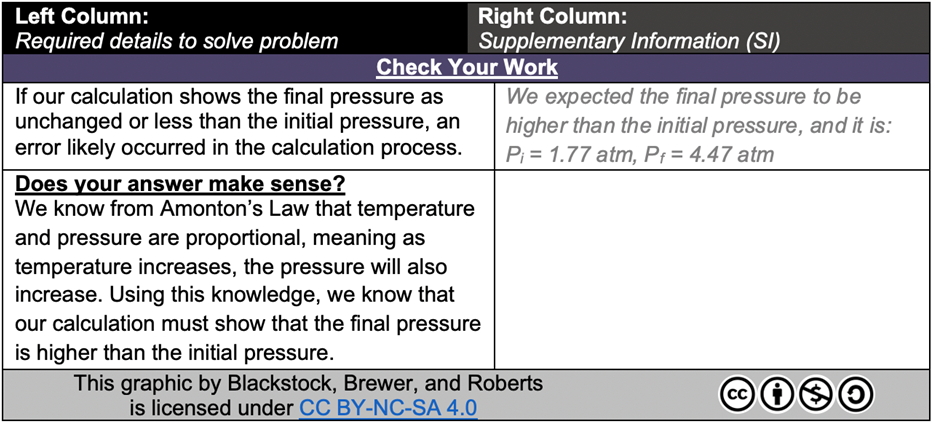
FIG. 8: “Check Your Work” excerpt from a completed PASS template example
As documented in Figure 8, if the answer attempt was incorrect, students can then decide how much additional support is required to understand how to solve the problem independently. Students may choose to navigate from the answer to one of the following sections that provide increasing levels of assistance: Strategy Map, Solution, or Guided Solution.
The final piece of the PASS template includes the authorship and attribution components for all the work, see Figure 9. This is a key piece that is often overlooked (Norris et al., 2023), even though the philosophy of open educational resources affords and advocates for attribution (Wiley, 2014). A common concern regarding the use/creation/adoption/adaptations of OER in academia is appropriate attributions and recognition of intellectual property (Otto, 2019).

FIG. 9: “Attribution” excerpt from a completed PASS template example. The resources used in the development of the completed PASS are clearly listed here. This promotes appropriate use of CC licensing and acknowledges intellectual property and creative contributions wherever possible/whenever available.
A recent review investigating opinions on the barriers and opportunities for adoption of OER in higher education (Menzli et al., 2022) demonstrated the importance of familiarity with academic copyrights and awareness of licenses. Often creators want to retain some recognition/rights for their contribution and may misunderstand Creative Commons licensing practices (Menzli et al., 2022; Tlili et al., 2022). West (2017) emphasized that attributions need to be correctly documented from the beginning when adopting or adapting OER; otherwise, material may not be able to be verified and thus cannot be used.
From an equity perspective, in Canadian contexts, Kayla Lar-Son emphasized that it is also essential to recognize how attribution connects to Canadian indigenous resource sharing (Centre for Teaching, Learning and Technology, University of British Columbia, 2022) and building relationships in connection with the initial artifact creation. As such, reciprocity was also considered as a key element of attribution within the first-year general chemistry classroom experience, in that it was important for chemistry educators who adapted materials, to attribute original relationships by also giving back to the knowledge commons, expanding upon and creating more OER in culturally appropriate ways.
Therefore, when the template was created, attribution documenting the question source history to recognize creators was the important final section.
The PASS template has the potential to serve multiple purposes. For the person who is creating the PASS solution, this framework organizes the details into “chunks” of required and supplementary information that provide clear increasing levels of support to the learner. Immersive engagement with this resource familiarizes students with the pattern of solving problems and illustrates that a consistent approach can be applied to work through various question types.
4.2 Adapting PASS
For most of our examples, we used our collection of first-year general chemistry PASS templates to illustrate topics across our postsecondary institution's articulated course learning outcomes, and we compared the current adaptations of the PASS template on two common platforms, LibreTexts and Pressbooks, which we will discuss below. However, as open advocates, we are aware of the limitations of open platforms to provide for all student design needs (Gupta & Belford, 2019; Martín-Sómer et al., 2024; Haleem et al., 2022). Some of the design concerns that became apparent included needing flexibility for diverse students with a variety of needs and preferences and a specific feedback loop design which recommends learners to use their first attempt to answer the question themself, then check to see if they need additional assistance to arrive at the correct answer.
In our experience, we found that the most useful open software platform tools (for content creation) were those that have underlying open values. We suggest open software that is designed for sharing from the beginning; has ubiquity in implementation and use; possesses adaptability with a focus on equity and accessibility; and is responsive to current software (e.g., LibreTexts and Pressbooks) and emerging trends (e.g., generative AI). Specifically, we suggest open software that is platform agnostic with a robustness of code and ability to transition between different modalities. We also support open software that promotes easy instructor changes and edits to adapt to cultural and pedagogical learning contexts.
4.3 Implementation in Digital Contexts
After developing the PASS template and a collection of completed PASS(es), the LibreTexts platform (https://chem.libretexts.org/) was selected for the first iteration of platform adaptation and implementation (see Figures 10–12). In addition, we used our local institutional incidence of the Pressbooks platform (https://pressbooks.com/) to host a collection of solutions split up as documented in Figures 13 and 14. In our context, these open platforms are open digital spaces in which we adapted and used the PASS framework. LibreTexts was used initially due to ease of use and simple design interface. There was a low usability barrier of entry for chemistry educators. Subsequent funding from an institutional TRU Open Press grant provided opportunity for professional learning support which ultimately facilitated a new design iteration in Pressbooks.

FIG. 10: A completed PASS adapted to the LibreTexts platform
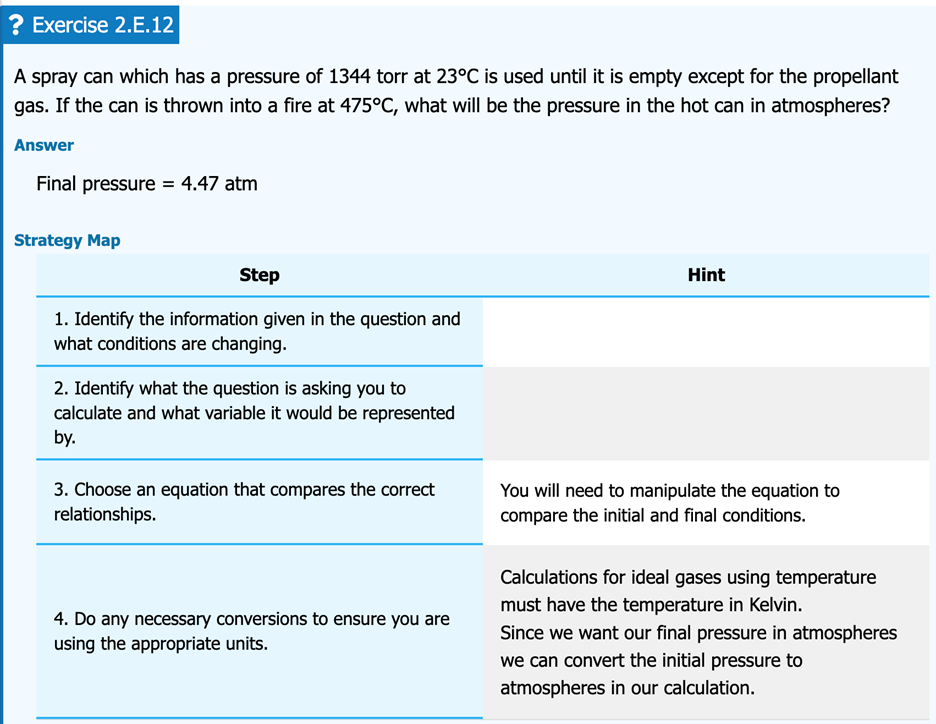
FIG. 11: The “Strategy Map” segment of the LibreTexts adapted PASS
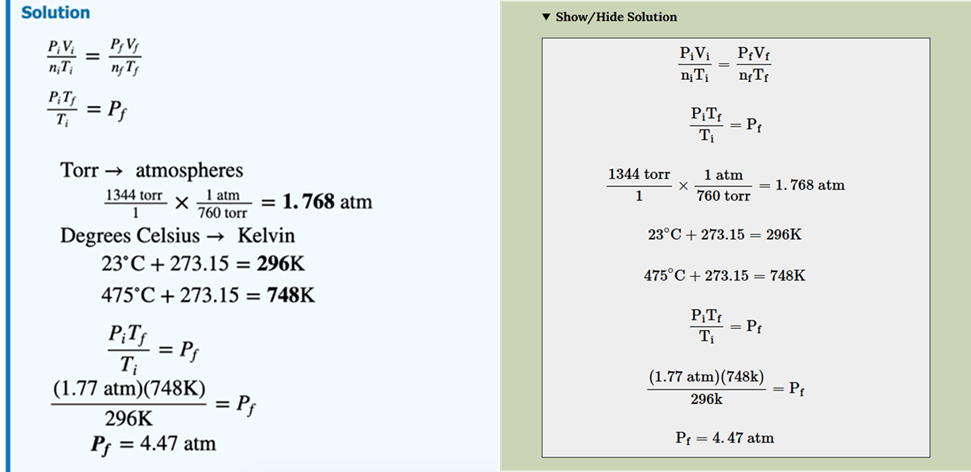
FIG. 12: The “Solution” segment of a completed PASS adapted for LibreTexts (left) and Pressbooks (right)

FIG. 13: The “Guided Solution” segment of a completed PASS adapted for Pressbooks. The supplementary information is currently “hidden.”

FIG. 14: The “Check Your Work” segment of a completed PASS adapted for Pressbooks. The supplementary information is currently “revealed.”
Figures 10–14 showcase excerpts of a completed PASS (Blackstock et al., n.d.; Brewer & Blackstock, n.d.) for a first-year general chemistry practice problem, to predict the impact of changing conditions of ideal gases. Despite subtle differences in the visual presentation of the information in each segment, the content delivered via either platform is the same and follows the guiding philosophies of the PASS approach detailed above.
In LibreTexts, the information in the PASS template was delivered in a linear format (Figure 10). Notice that the learner is initially presented only with the question and has the option to reveal the answer, strategy map, solution, or guided solution. In this iteration of PASS, the Check Your Work section is hidden within the guided solution, which when clicked on reveals more content.
Once revealed, each segment of the LibreTexts adapted solution (i.e., answer, strategy map, solution, and guided solution) closely resembles the blank PASS template, with required content housed within a column on the left-hand side paired with supplementary information, such as hints and links to other learning resources in the right-hand column of the same row (Figure 11).
In comparison, rather than presenting the hints to the right, the strategy map segment in the Pressbooks iteration (not shown) keeps the content of the hints “hidden” and can be similarly “revealed” by clicking on the bolded word, see Figure 13. In both adaptations, the strategy map organized each procedural item in the approach into discrete steps. If the learner applies the suggested strategy to the information provided in the question, they can formatively compare their work with the “solution” segment that immediately follows (Figure 12).
The solution is presented in a linear format, ensuring that no steps are left out. This is consistent in both LibreTexts and Pressbooks platforms (Figure 12). Some proficiency in HTML coding and/or use of related coding tools and technologies was required to translate each mathematical step appropriately from the original solution template into this open online format.
If the learner does not understand/cannot rationalize/cannot explain/fails to reconcile the suggested steps in the strategy map with the line-by-line solution, they are encouraged to read through the guided solution. In LibreTexts, this segment is again formatted similarly to the PASS template (see Figure 6).
In the Pressbooks iteration, each supplementary, clickable “hint” has been sorted into four categories based on the type of additional information that is being provided (see Figure 13).
The content contained within the guided solution is intended to be comprehensive so it can provide an approachable pathway to understanding for all learners, regardless of their level of competency. The descriptions contextualize each step of the approach with respect to the disciplinary specific theory. Proficient integration of theory with the algorithmic problem-solving techniques is required to successfully complete the problems and can also be used to confidently predict the answer to many chemistry problems.
In this section, the figures were able to exemplify and describe the adaptation of the PASS framework in multiple contexts. A learner who uses a digital artifact created with the PASS framework has freedom to choose which segments they refer to and how much of the supplementary information they engage with. Neither the LibreTexts or Pressbooks platform restrict the learner when interacting with the content in a sequential or fixed order. Each user has the flexibility to alternate/oscillate between each level of support as needed, depending on their familiarity with each type of practice problem, making this tool appropriate for a range of learners.
5. CONCLUSIONS AND FUTURE CONSIDERATIONS AND WORK
In this article we present the PASS framework, which can be used to support students in completing problems by providing multiple resources and formative assessment to ensure students have the opportunity to self-check their own learning. The primary use of the framework in our context was to address identified gaps in existing first-year general chemistry student formative assessment practices. We highlight the importance of focusing on formative assessment in first-year general chemistry in higher education contexts. The literature-based rationale is described by considering current learning theory in connection with OEPs and open homework systems. The article concludes with examples of how to use the framework as a learner and why an educator might want to consider using it in specific contexts and with specific platforms. A collection of PASS examples and their adaptation to two open platforms (Pressbooks and LibreTexts) are showcased, and the utility and execution of the PASS templates were compared. Furthermore, we have demonstrated the adaptability of the PASS template to different platforms (i.e., LibreTexts and Pressbooks) and flexibility of implementation across diverse modalities of delivery, ranging from simple PDF printable worksheets to increasingly interactive H5P or LMS quizzes and interactive videos.
The PASS framework was ultimately developed to fit the niche requirement of an inclusive, user-friendly, scaffolded solution approach to support students in their studies outside the classroom. The framework itself had underlying design principles of supporting all learners through a lens of UDL equitability, immersive scaffolding, and building self-confidence that prioritizes providing opportunities for student choice. The specific strategies implemented in terms of putting UDL in place could include alt-text, screen reader optimization, low-bandwidth versions, and multilingual access. Accessibility needs were considered and implemented across the various platforms where feasible. Using the framework, we developed comprehensive PASS solutions that could be used for reflective formative self-assessment practice exercises.
The collected comprehensive solutions were primarily developed from questions from existing OER. We used available first-year general chemistry practice problems to support the aligned curriculum and to create new questions to expand upon missing topics. These new solutions have been shared openly (as per CC-BY-NC-SA) on multiple platforms. We hope that other educators may adopt or adapt this platform resource for their students and openly share our collected chemistry solutions. We aim to continue to create and compile completed PASSs to cover the various topics and key learning outcomes essential for student success in first-year general chemistry.
The PASS framework was developed to be multidisciplinary and support students in improving their skills by using it as a formative self-assessment process while solving problems outside the classroom. While the framework has been implemented for first-year general chemistry, we believe it will be an effective tool for STEM educators and may also be used by educators from other fields to organize discipline-specific solutions for their students.
5.1 Future Considerations
The next steps for our general chemistry team are to consider the evaluation, perception, usage and impact of PASS adaptations, which will include obtaining the research ethics approval of data collection from our users. We hope to continue to develop and enhance our current collection of chemistry PASS practice examples. In addition, we will increase our knowledge mobilization by promoting and disseminating the collection for adoption by other chemistry educators and students beyond the TRU audience.
Beyond our immediate context, we aim to share our framework to enhance and improve other disciplinary adaptations and encourage other disciplines to adopt and adapt our framework for use in their course design and learning materials. Finally, we hope to find creative and innovative approaches to merge our framework and design to enhance other emerging technologies, such as efficient strategies to effectively prompt generative artificial intelligence (GenAI) responses and investigate the application of GenAI tools which integrate with the PASS framework. By promoting, expanding, and adapting PASS, we hope to streamline the creation of additional equitable, accessible, and responsive PASS projects in various disciplines and applications.
ACKNOWLEDGMENTS
We would like to acknowledge the support of the following people and organizations in the creation of the template and the adaptations presented in this article and helping us with the PASS project: TRU Open Press, LibreTexts, Delmar Larsen, Ashlynn Jensen, C. Esther Ojukwu, Dani Collins, Kaitlyn Meyers, and Jessica Obando Almache. The PASS content development was made possible by funding from both TRU Open Press Grant and a TRU Instructional Innovation Grant (TIIG). The PASS framework development was funded by a TRU Open Educational Resources Development Grant.
REFERENCES
Algar, W. R., Elouazizi, N., Stewart, J. J., Maxwell, E. J., Tan, T., Zhang, Z., Stoodley, R., Rodríguez Núñez, J. R., Terpstra, A. S., & Wickenden, J. G. (2022). The Alchemy Project: A personalized, flexible, and scalable active learning platform to kelp foster expert-like thinking in chemistry. Journal of Chemical Education, 99(9), 3104–3113. https://doi.org/10.1021/acs.jchemed.2c00097
Almeqdad, Q. I., Alodat, A. M., Alquraan, M. F., Mohaidat, M. A., & Al-Makhzoomy, A. K. (2023). The effectiveness of universal design for learning: A systematic review of the literature and meta-analysis. Cogent Education, 10(1), 2218191. https://doi.org/10.1080/2331186X.2023.2218191
Barr, D. A., Matsui, J., Wanat, S. F., & Gonzalez, M. E. (2010). Chemistry courses as the turning point for premedical students. Advances in Health Sciences Education, 15(1), 45–54. https://doi.org/10.1007/s10459-009-9165-3
Blackstock, L., Brewer, S., & Jensen, A. (n.d.). PASS Chemistry Book CHEM 1510/1520. Chemistry LibreTexts. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1510_1520
Block, J. H., & Burns, R. B. (1976). Mastery learning. Review of Research in Education, 4(1), 3–49. https://doi.org/10.3102/0091732X004001003
Bowman, C. R., Gulacar, O., & King, D. B. (2014). Predicting student success via online homework usage. Journal of Learning Design, 7(2), 47–61. https://doi.org/10.5204/jld.v7i2.201
Brewer, S., & Blackstock, L. (n.d.). PASSchem: Platform adapted strategic solutions: Comprehensive solutions for general chemistry practice questions. TRU Open Press. Retrieved August 15, 2024, from https://passchem.pressbooks.tru.ca/
Buhr, S. P. (2020). 21st century physics homework: A mixed-methods approach evaluating how an individualized online homework platform can provide quality feedback and help physics students engage in self-regulated learning. [Doctoral dissertation, University of South Carolina]. Scholar Commons Repository. https://scholarcommons.sc.edu/etd/6051
Butler, D. L., & Winne, P. H. (1995). Feedback and self-regulated learning: A theoretical synthesis. Review of Educational Research, 65(3), 245–281. https://doi.org/10.3102/00346543065003245
Centre for Teaching, Learning and Technology, University of British Columbia. (2022, March 22). The 6R's of Indigenous OER: Re imagining OER to Honour Indigenous Knowledge and Sovereignty [Video]. YouTube. https://www.youtube.com/watch?v=Lwciwt-gXoQ
Chin, C. (2007). Teacher questioning in science classrooms: Approaches that stimulate productive thinking. Journal of Research in Science Teaching, 44(6), 815–843. https://doi.org/10.1002/tea.20171
Cracolice, M. S., Deming, J. C., & Ehlert, B. (2008). Concept learning versus problem solving: A cognitive difference. Journal of Chemical Education, 85(6), Article 873. https://doi.org/10.1021/ed085p873
Craig, C. D. (2020). 2020 pandemic: Resilient Canadian higher education institutions will integrate OER. In N. Callaos, B. Muirhead, L. Robertson, B. Sanchez, & M. Savoie (Eds.), Proceedings of the 14th International Multi-Conference on Society, Cybernetics and Informatics (IMSCI 2020) (pp. 149–153). Curran Associates. https://www.iiis.org/CDs2020/CD2020Summer/papers/EA596LT.pdf
Cronin, C. (2017). Openness and praxis: Exploring the use of open educational practices in higher education. The International Review of Research in Open and Distributed Learning, 18(5). https://doi.org/10.19173/irrodl.v18i5.3096
Crossouard, B., & Pryor, J. (2012). How theory matters: Formative assessment theory and practices and their different relations to education. Studies in Philosophy and Education, 31(3), 251–263. https://doi.org/10.1007/s11217-012-9296-5
Eichler, J. F., & Peeples, J. (2013). Online homework put to the test: A report on the impact of two online learning systems on student performance in general chemistry. Journal of Chemical Education, 90(9), 1137–1143. https://doi.org/10.1021/ed3006264
European Commission: Directorate-General for Education, Youth, Sport and Culture, Cefai, C., Downes, P., & Cavioni, V. (2021). A formative, whole-school approach to the assessment of social and emotional education in the EU: Executive summary. Publications Office of the European Union. https://data.europa.eu/doi/10.2766/905981
Fink, A., Cahill, M. J., McDaniel, M. A., Hoffmann, A., & Frey, R. F. (2018). Improving general chemistry performance through a growth mindset intervention: Selective effects on underrepresented minorities. Chemistry Education Research and Practice, 19(3), 783–806. https://doi.org/10.1039/C7RP00244K
Fink, A., Frey, R. F., & Solomon, E. D. (2020). Belonging in general chemistry predicts first-year undergraduates' performance and attrition. Chemistry Education Research and Practice, 21(4), 1042–1062. https://doi.org/10.1039/D0RP00053A
Flanders, G. R. (2017). The effect of gateway course completion on freshman college student retention. Journal of College Student Retention: Research, Theory & Practice, 19(1), 2–24. https://doi.org/10.1177/1521025115611396
Gilbert, D. (2017, May). Think about the chemistry, not the math. Chem 13 News Magazine. https://uwaterloo.ca/chem13-news-magazine/may-2017/feature/think-about-chemistry-not-math
Gondra, N., Suresh, S., Anilao, A., Toy, S., Pfile, S. B., Vernoy, B. J., Lee, J. M. Y., Raghavendra, R., Ravi, A., & Gulacar, O. (2024). Analyzing the impact of time spent on practice questions on general chemistry students' problem-solving performance. Journal of Chemical Education, 101(8), 3072–3084. https://doi.org/10.1021/acs.jchemed.4c00207
Gupta, T., & Belford, R. E. (2019). Conclusion: Technology integration in chemistry education and research: What did we learn and what can we expect going forward? In Technology integration in chemistry education and research (TICER) (pp. 281–301). American Chemical Society. https://doi.org/10.1021/bk-2019-1318.ch018
Haleem, A., Javaid, M., Qadri, M. A., & Suman, R. (2022). Understanding the role of digital technologies in education: A review. Sustainable Operations and Computers, 3, 275–285. https://doi.org/10.1016/j.susoc.2022.05.004
Harris, R. B., Mack, M. R., Bryant, J., Theobald, E. J., & Freeman, S. (2020). Reducing achievement gaps in undergraduate general chemistry could lift underrepresented students into a “hyperpersistent zone.” Science Advances, 6(24), eaaz5687. https://doi.org/10.1126/sciadv.aaz5687
Hattie, J., & Timperley, H. (2007). The power of feedback. Review of Educational Research, 77(1), 81–112. https://doi.org/10.3102/003465430298487
Jhangiani, R. S., & Jhangiani, S. (2017). Investigating the perceptions, use, and impact of open textbooks: A survey of post-secondary students in British Columbia. The International Review of Research in Open and Distributed Learning, 18(4). https://doi.org/10.19173/irrodl.v18i4.3012
Jhangiani, R., S., Pitt, R., Hendricks, C., Key, J., & Lalonde, C. (2016). Exploring faculty use of open educational resources at British Columbia post-secondary institutions. BCcampus Research Report. BCcampus. https://open.bccampus.ca/files/2016/04/BCFacultyUseOfOER_final.pdf
Johnson, R. H. (2022). Instructor talk and its effects on the science identity of female high school students (Publication No. 2663465550) [Doctoral dissertation, University of Arkansas at Little Rock]. ProQuest Dissertations & Theses.
Johnston, A. C. (2020). Teacher talk in engineering design projects. [Doctoral dissertation, Purdue University]. Hammer Research Repository. https://doi.org/10.25394/PGS.12194277.v1
Khoza, S. B. (2016). Is teaching without understanding curriculum visions and goals a high risk? South African Journal of Higher Education, 30(5), 104–119. https://doi.org/10.20853/30-5-595
Killi, S., & Morrison, A. (2015). Just-in-time teaching, just-in-need learning: Designing towards optimized pedagogical outcomes. Universal Journal of Educational Research, 3(10), 742–750. https://doi.org/10.13189/ujer.2015.031013
Knowles, M. S. (1975). Self-directed learning: A guide for learners and teachers. Association Press.
Koch, A. K. (2017). It's about the gateway courses: Defining and contextualizing the issue. New Directions for Higher Education, 2017(180), 11–17. https://doi.org/10.1002/he.20257
Lalonde, C. (2020, May 7). The cost of homework. BCcampus. https://bccampus.ca/2020/05/07/the-cost-of-homework/
Lantrip, J., & Ray, J. (2020). Faculty perceptions and usage of OER at Oregon Community Colleges. Community College Journal of Research and Practice, 45(12), 896–910. https://doi.org/10.1080/10668926.2020.1838967
Lee, E. N., & Orgill, M. (2022). Toward equitable assessment of English language learners in general chemistry: Identifying supportive features in assessment items. Journal of Chemical Education, 99(1), 35–48. https://doi.org/10.1021/acs.jchemed.1c00370
Leinhardt, G., Cuadros, J., & Yaron, D. (2007). “One firm spot”: The role of homework as lever in acquiring conceptual and performance competence in college chemistry. Journal of Chemical Education, 84(6), 1047. https://doi.org/10.1021/ed084p1047
Lipscomb, L., Swanson, J., & West, A. (2010). Scaffolding. In M. Orey (Ed.), Emerging perspectives on learning, teaching, and technology (pp. 226-238). Global Text Project.
Mahalingam, M., Morlino, E. A., & Fasella, E. (2019). The impact of technology-assisted “scaffolding” on student learning in general chemistry. In Technology Integration in Chemistry Education and Research (TICER) (pp. 233–245). American Chemical Society. https://doi.org/10.1021/bk-2019-1318.ch015
Mandouit, L., & Hattie, J. (2023). Revisiting “The Power of Feedback” from the perspective of the learner. Learning and Instruction, 84, Article 101718. https://doi.org/10.1016/j.learninstruc.2022.101718
Marín, V. I., Peters, L. N., & Zawacki-Richter, O. (2022). (Open) educational resources around the world: An international comparison. EdTech Books. https://edtechbooks.org/oer_around_the_world
Martín-Sómer, M., Casado, C., & Gómez-Pozuelo, G. (2024). Utilising interactive applications as educational tools in higher education: Perspectives from teachers and students, and an analysis of academic outcomes. Education for Chemical Engineers, 46, 1–9. https://doi.org/10.1016/j.ece.2023.10.001
Menzli, L. J., Smirani, L. K., Boulahia, J. A., & Hadjouni, M. (2022). Investigation of open educational resources adoption in higher education using Rogers' diffusion of innovation theory. Heliyon, 8(7), e09885. https://doi.org/10.1016/j.heliyon.2022.e09885
Nabulsi, L., Nguyen, A., & Odeleye, O. (2021). A comparison of the effects of two different online homework systems on levels of knowledge retention in general chemistry students. Journal of Science Education and Technology, 30(1), 31–39. https://doi.org/10.1007/s10956-020-09872-2
Norris, M. E., Swartz, M., & Kuhlmeier, V. A. (2023). The importance of copyright and shared norms for credit in open educational resources. Frontiers in Education, 7. https://doi.org/10.3389/feduc.2022.1069388
Otto, D. (2019). Adoption and diffusion of open educational resources (OER) in education: A meta-analysis of 25 OER-projects. The International Review of Research in Open and Distributed Learning, 20(5), 122–140. https://doi.org/10.19173/irrodl.v20i5.4472
Parmigiani, D., Nicchia, E., Murgia, E., & Ingersoll, M. (2024). Formative assessment in higher education: An exploratory study within programs for professionals in education. Frontiers in Education, 9. https://doi.org/10.3389/feduc.2024.1366215
Powell, C. B., Simpson, J., Williamson, V. M., Dubrovskiy, A., Walker, D. R., Jang, B., Shelton, G. R., & Mason, D. (2020). Impact of arithmetic automaticity on students' success in second-semester general chemistry. Chemistry Education Research and Practice, 21(4), 1028–1041. https://doi.org/10.1039/D0RP00006J
Richards-Babb, M., Curtis, R., Ratcliff, B., Roy, A., & Mikalik, T. (2018). General chemistry student attitudes and success with use of online homework: Traditional-responsive versus adaptive-responsive. Journal of Chemical Education, 95(5), 691–699. https://doi.org/10.1021/acs.jchemed.7b00829
Richards-Babb, M., & Jackson, J. K. (2011). Gendered responses to online homework use in general chemistry. Chemistry Education Research and Practice, 12(4), 409–419. https://doi.org/10.1039/C0RP90014A
Roberts, V. (2022). Open learning design for using open educational practices in high school learning contexts and beyond. Journal for Multicultural Education, 16(5), 491–507. https://doi.org/10.1108/JME-01-2022-0019
Ryu, M., Bano, R., & Wu, Q. (2021). Where does CER stand on diversity, equity, and inclusion? Insights from a literature review. Journal of Chemical Education, 98(12), 3621–3632. https://doi.org/10.1021/acs.jchemed.1c00613
Saba, F., & Shearer, R. L. (2017). Transactional distance and adaptive learning: Planning for the future of higher education. Routledge. https://doi.org/10.4324/9780203731819
Sadler, D. R. (1998). Formative assessment: Revisiting the territory. Assessment in Education: Principles, Policy & Practice, 5(1), 77–84. https://doi.org/10.1080/0969595980050104
Salehi, S., Cotner, S., & Ballen, C. J. (2020). Variation in incoming academic preparation: Consequences for minority and first-generation students. Frontiers in Education, 5. https://doi.org/10.3389/feduc.2020.552364
Salame, I. I., & Hanna, E. (2020). Studying the impact of online homework on the perceptions, attitudes, study habits, and learning experiences of chemistry students. Interdisciplinary Journal of Environmental and Science Education, 16(4), e2221. https://doi.org/10.29333/ijese/8543
Siegel, M. A., Wissehr, C., & Halverson, K. (2008, March). Sounds like success: A framework for equitable assessment. The Science Teacher, 75(3), 43–46.
Shi, L., Wang, Y., K. Mitchell-Jones, J., & Stains, M. (2024). Why do we assess students? Investigating general chemistry instructors' conceptions of assessment purposes and their relationships to assessment practices. Chemistry Education Research and Practice, 25(4), 1159–1174. https://doi.org/10.1039/D4RP00147H
Stone, D. C. (2021). Student success and the high school-university transition: 100 years of chemistry education research. Chemistry Education Research and Practice, 22(3), 579–601. https://doi.org/10.1039/D1RP00085C
Tashiro, J., & Talanquer, V. (2021). Exploring inequities in a traditional and a reformed general chemistry course. Journal of Chemical Education, 98(12), 3680–3692. https://doi.org/10.1021/acs.jchemed.1c00821
Taylor, C., & Taylor, M. W. (2018). I'm never doing this again!: identifying and solving faculty challenges in adoption of open educational resources. Online Journal of Distance Learning Administration, 21(2). https://www.learntechlib.org/p/188453/
Tlili, A., Altinay, F., Huang, R., Altinay, Z., Olivier, J., Mishra, S., Jemni, M., & Burgos, D. (2022). Are we there yet? A systematic literature review of Open Educational Resources in Africa: A combined content and bibliometric analysis. PLOS ONE, 17(1), e0262615. https://doi.org/10.1371/journal.pone.0262615
Tsaparlis, G. (2021). Introduction: The many types and kinds of chemistry problems. In G. Tsaparlis, G. (Ed.), Problems and problem solving in chemistry education: Analysing data, looking for patterns and making deductions (pp. 1–14). Royal Society of Chemistry. https://doi.org/10.1039/9781839163586
Wang, Y., Apkarian, N., Dancy, M. H., Henderson, C., Johnson, E., Raker, J. R., & Stains, M. (2024). A national snapshot of introductory chemistry instructors and their instructional practices. Journal of Chemical Education, 101(4), 1457–1468. https://doi.org/10.1021/acs.jchemed.4c00040
West, Q. (2017). Librarians in the pursuit of open practices. In R. S. Jhangiani & R. Biswas-Diener (Eds.), Open: The Philosophy and Practices that are Revolutionizing Education and Science (pp. 139–146). Ubiquity Press. https://www.jstor.org/stable/j.ctv3t5qh3
Weston, T. J., Seymour, E., Koch, A. K., & Drake, B. M. (2019). Weed-out classes and their consequences. In E. Seymour & A.-B. Hunter (Eds.), Talking about leaving revisited: Persistence, relocation, and loss in undergraduate STEM education (pp. 197–243). Springer International Publishing. https://doi.org/10.1007/978-3-030-25304-2_7
Wiley, D. (2014, March 5). The access compromise and the 5th R. Improving learning. https://opencontent.org/blog/archives/3221
Williams, K. M., & Wendler, C. (2020). The open admissions model: An example from the United States. In M. E. Oliveri & C. Wendler (Eds.), Higher education admissions practices: An international perspective (pp. 51–75). Cambridge University Press. https://doi.org/10.1017/9781108559607.004
Wilson, E. E., & Kennedy, S. A. (2017). Modern “homework” in general chemistry: An extensive review of the cognitive science principles, design, and impact of current online learning systems. In Online approaches to chemical education (pp. 101–130). American Chemical Society. https://doi.org/10.1021/bk-2017-1261.ch009
Wood, C. (2006). The development of creative problem solving in chemistry. Chemistry Education Research and Practice, 7(2), 96–113. https://doi.org/10.1039/B6RP90003H
Yuriev, E., Naidu, S., S. Schembri, L., & L. Short, J. (2017). Scaffolding the development of problem-solving skills in chemistry: Guiding novice students out of dead ends and false starts. Chemistry Education Research and Practice, 18(3), 486–504. https://doi.org/10.1039/C7RP00009J
Comments
Show All Comments
Change Subscription
Information
Server Error